
You've reached the Virginia Cooperative Extension Newsletter Archive. These files cover more than ten years of newsletters posted on our old website (through April/May 2009), and are provided for historical purposes only. As such, they may contain out-of-date references and broken links.
To see our latest newsletters and current information, visit our website at http://www.ext.vt.edu/news/.
Newsletter Archive index: http://sites.ext.vt.edu/newsletter-archive/

Fetal Pig Programming - An Emerging Concept with Possible Implications for Swine Reproductive Performance
Livestock Update, April 2008
Mark J. Estienne and Allen F. Harper, VA Tech Tidewater AREC, Suffolk, VA
![]()
Introduction
Consider for a moment the life of replacement gilts from birth to the point at which they farrow their first litters of pigs. At any point within that spectrum of time, modern swine production has benefited from many years of research attempting to define the optimum environment (number of pen-mates, size of pens, temperature, etc.) in which gilts are raised so as to ultimately maximize their reproductive efficiency. For example, litter size in which gilts are raised impacts the size of the litters that they produce. Nelson and Robison (1976) reported the results of an experiment during which litter size was standardized at either six or twelve pigs. Later in life, gilts from the small litters had more ovulations and embryos at 25 days post-mating compared to gilts from the large litters; Gilts that farrowed averaged over one more pig born alive. These data suggest that larger litter size imposes some type of “stress” pre-weaning that negatively impacts a female’s future reproduction. This potential negative effect can be addressed by strategic cross-fostering.
The post-weaning environment in which gilts are raised can ultimately impact reproduction as well. For example, in an experiment conducted at the Tidewater AREC (Lindemann et al., 1988), the percentage of gilts reaching puberty at less than 285 days of age tended to be greater for females allowed adequate floor space during the grower and finisher phases of production, compared to gilts allowed less floor space (6 versus 3 ft2 during the grower phase, and 7.8 vs. 6 ft2 during the finisher phase). Moreover, Kuhlers et al. (1985) placed grower gilts in pens of 8 or 16 animals each. Gilts reared in the smaller groups ultimately farrowed one more pig per litter than did gilts reared in larger groups.
Thus, an enormous amount of research has been conducted to determine the effect of various environmental factors to which gilts are exposed from birth onward, with reproductive capacity they ultimately achieve as the measured endpoint. Receiving far less attention, however, is another period of time during which “environmental” factors may impact subsequent gilt reproduction. Indeed, an exciting and growing body of evidence supports the notion that the maternal environment in which gilt fetuses develop plays a profound role in the development of the reproductive and other physiologic systems.
The objectives of this paper are to provide the reader with a brief introduction to the concept of “fetal programming” and then describe how this phenomenon possibly relates to a very contentious issue facing the swine industry that being the housing of gestating sows.
Prenatal Development and Fetal Programming in the Pig
Fertilization by sperm cells of the ova (or “eggs”) released by sows or gilts during the process of ovulation occurs in the oviduct a few hours after mating. Cell division begins soon after and the fertilized egg passes into the uterus by the third day post-mating. Cell specialization and rearrangement begins by the sixth day. Eleven day-old embryos begin to show signs of attachment to the lining of the uterus and true implantation and formation of the placenta occurs around day 18. By this time within the embryo the ectoderm, mesoderm, and entoderm are clearly formed and cell specialization continues. From the ectoderm arise the skin, mammary and sweat glands, hair and hoofs, the intestinal lining, teeth enamel and the nervous system. From the entoderm arise components of the digestive tract, thyroid gland, trachea, and lungs. From the mesoderm arise the skeleton, skeletal muscle, connective tissue, blood vessels, blood cells, heart, smooth muscle, adrenal glands, reproductive organs, and the kidneys. Many of the major organs can be seen by day 20 post-mating. The size of the developing fetuses increases tremendously during the last half of the gestation period. Shown in Table 1 are some important chronological events in the prenatal growth of female swine, and emphasize the development of reproductive organs.
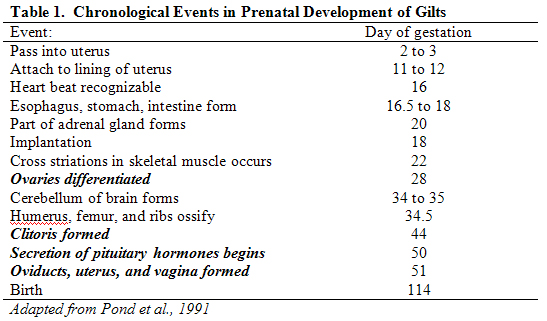
Fetal programming refers to the process by which an acute or chronic stimulus in utero (i.e., in the uterus) establishes a permanent response in the fetus that impacts physiologic function later in life. When reviewing the time course of fetal development of the pig described in the preceding paragraph and Table 1, it becomes intuitive that depending on the nature and timing of the stimulus, various physiological systems can be differentially impacted. The concept of fetal programming in swine is illustrated by an experiment reported by O’Gorman et al. (2007). In that study, gestating crossbred sows were allocated to one of two treatment groups: control or stressed. “Stressed” sows were subjected to daily restraint for five minutes during weeks 12 to 16 of gestation. Female offspring were checked for estrus twice daily beginning at 122 days of age. Age at first estrus was significantly delayed in gilts farrowed by stressed sows (172 + 6 days) compared to gilts farrowed by control females (158 + 2 days).
Does the Type of Housing in which Sows Gestate Impact Subsequent Reproduction in Gilt Offspring?
Individually housing pregnant females results in certain production advantages, and it is estimated that at least 60% of the sows and gilts in the U.S. are kept in stalls throughout gestation (Barnett et al., 2001). This method of sow housing, however, is the most contentious welfare issue facing pork producers. Typical gestation stalls measure 2’ x 7’ and limit sows to standing, sitting, and lying. This restricted freedom of movement has been, and continues to be, robustly criticized by animal rights and animal welfare activists who proclaim that gestation stalls are inherently stressful and do not provide for sow well-being. On the basis of a comprehensive review of the scientific literature, however, McGlone et al. (2004) concluded that well-managed stalls or group pens generally produce similar states of well-being for pregnant sows in terms of physiology, behavior, performance, and health. Results from research conducted at the Tidewater AREC (Estienne et al., 2006) generally support this conclusion.
We conducted an experiment utilizing a total of 56 gilts, which compared pregnancy rates and the number of embryos present 30 days post-mating in females group-housed in pens of three or housed individually in gestation stalls. Between groups, there were no differences in the proportion of animals displaying stereotypies, defined as repeated movements, oral activities without obvious finality, rooting and nosing. Group-housed gilts gained significantly more weight than did gilts housed in stalls. Injury scores and the incidence of lameness were significantly greater in gilts housed in groups, but serum concentrations of cortisol, a classical “stress” hormone tended to be greater in gilts housed in stalls (79.4 versus 57.1 ng/mL; SE = 7.8). Pregnancy rate was higher for gilts housed in stalls (100%) compared with group-housed individuals (85.7%); however, there was no effect of treatment on the number of embryos recovered.
We are now in the midst of an investigation funded in 2007 by the Virginia Pork Industry Board, the objective of which is to determine if the type of housing in which sows gestate impact subsequent reproduction in gilt offspring. Our working hypothesis is that if there is indeed a difference between housing systems (individual stalls versus group pens) in terms of “stress” to, and well-being of, the sow, then due to fetal programming, growth and reproductive performance of gilt offspring may be impacted.
Gilts were mated by artificial insemination (AI) and allotted to one of three types of gestation housing: I. individual stalls throughout gestation, II. group pens throughout gestation (5 to 6 gilts/pen); or III. individual stalls for 30 days post-mating and then group pens for the remainder of gestation. At day 110 of gestation, gilts were moved to the farrowing barn. Data for the experimental females is contained in Table 2 and graphically depicted in Figure 1. Barrow pigs were cross-fostered among litters within a treatment group so that sows were nursing an approximately equal number of pigs (10.5 + 0.3).
Table 2. Farrowing data for females that were kept in individual stalls or group pens throughout gestation or that were
kept in individual stalls for the first 30 days after mating and then group pens for the remainder of gestation.
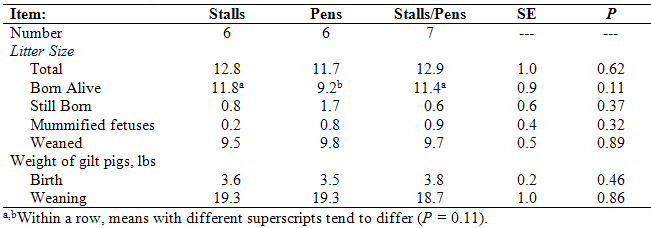
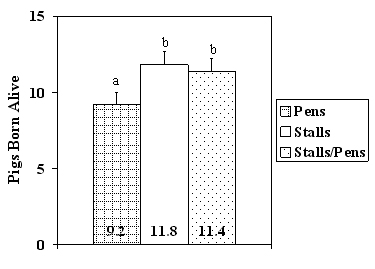
Figure 1. Pigs born alive for gilts housed in group pens for the entire gestation period, stalls for the entire
gestation period, or stalls for the first 30 days post-mating and then group pens for the remainder
of gestation (n = 6 to 7 gilts per treatment). Bars with different superscripts tend to differ (P = 0.11).
There were no significant effects of treatment on litter size, although there was a trend for a greater number of pigs born alive for females kept in stalls throughout gestation or in stalls for the first thirty days post-mating and group pens for the remainder of pregnancy, compared to gilts kept in group pens throughout gestation. The body weights of gilts pigs were similar at birth and at weaning (24.6 + 0.3 days of age) among treatments.
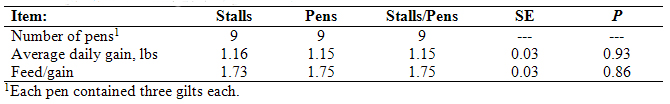
At weaning, gilts were placed in nursery pens each containing three pigs farrowed exclusively by gilts exposed to one of the three gestation housing systems described above. During the 5-week nursery phase of the study, average daily gain and feed conversion efficiency were similar among groups (Table 3). Thus, piglet growth during the lactation and nursery phases was unaffected by the type of gestation housing to which the dams were exposed. This suggests that if gestation housing does indeed affect gilt offspring performance via fetal programming, the effects are manifested later in life and not during early postnatal growth. Consistent with this hypothesis, in a review of literature, Foxcroft and Town (2004) concluded that variation in growth performance after birth is pre-programmed during fetal development in the uterus and it is likely that these pre-programmed limitations in growth performance express themselves in the late grower or early finisher stages of production.
Table 3. Nursery performance for gilts farrowed by females that were kept in individual stalls or group pens
throughout gestation, or individual stalls for the first 30 days after mating and then group pens for the remainder of gestation.
After the nursery stage of production, intact pens of gilts were moved to the growing-finishing building and growth performance is currently being assessed. The grow-finish phase of the research will end when animals reach 240 lbs of body weight. Beginning at 5.5 months of age, gilts will be checked daily for estrus and age at puberty determined. Gilts will be mated using AI and at 30 days post-mating, humanely killed and reproductive tracts collected. Pregnancy status, number of corpora lutea (i.e., ovulation rate), total number of embryos, number of viable embryos, embryo length and weight, and percent embryo survival will be determined.
Summary
Fetal programming refers to the process by which an acute or chronic stimulus in the uterus establishes a permanent response within the fetus that impacts physiologic function later in life. This phenomenon occurs in swine as illustrated by the fact that subjection of sows to five minutes of daily restraint during weeks 12 to 16 of gestation delays the onset of puberty in gilt offspring. Ongoing research at the Tidewater AREC is testing the hypothesis that performance of gilt offspring is impacted by the type of housing (stalls and/or pens) in which their maternal sows are kept during gestation. At the conclusion of our study, we will also have data to either support or refute the argument that the well-being of sows (as assessed by performance of gilt offspring) is compromised by gestation stall housing. This study will offer insight into the emerging concept of fetal pig programming and findings could impact future housing systems.
Literature Cited
Barnett, J.L., P.H. Hemsworth, G.M. Cronin, E.C. Jongman, and G.D. Hutson. 2001. A review of the welfare issues for sows and piglets in relation to housing. Australian Journal of Agricultural Research 52:1-28.
Estienne, M.J., and A.F. Harper. 2006. Reproductive traits in gilts housed individually or in groups during the first thirty days of gestation. Journal of Swine Health and Production 14:241-246.
Foxcroft, G.R., and S.C. Town. 2004. Prenatal programming of postnatal performance - The unseen cause of variance. Advances in Pork Production 15:269-279.
Kuhlers, D.L., S.B. Jungst, D.N. Marple, and C.H. Rahe. 1985. The effect of pen density during rearing on subsequent reproductive performance in gilts. Journal of Animal Science 61:1066-1069.
Lindemann, M.D., E.T. Kornegay, and E. van Heugten. 1987-1988. Influence of stocking density on performance and immune response of swine. Virginia Tech Livestock Research Report No. 7:185-188.
McGlone, J.J., E.H. von Borell, J. Deen, A.K. Johnson, D.G. Levis, M. Meunier-Salaun, J. Morrow, D. Reeves, J.L. Salk-Johnson, and P.L. Sundberg. 2004. Review: Compilation of the scientific literature comparing housing systems for gestating sows and gilts using measures of physiology, behavior, performance, and health. Professional Animal Scientist 20:105-117.
Nelson, R.E., and O.W. Robison. 1976. Effects of postnatal maternal environment on reproduction of gilts. Journal of Animal Science 43:71-77.
O’Gorman, C.W., E. Gonzales, M.D. Eaton, K.A. Collard, M. Reyna, J.C. Laurenz, R.L. Stanko, D.H. Keisler, J.A. Carroll, and M.R. Garcia. 2007. Fetal exposure to maternal stress influences leptin receptor gene expression during development and age at puberty in gilts. Journal of Animal Science 85(Suppl. 2):13.
Pond, W.G., J.H. Maner, and D.L. Harris. 1991. In: Pork Production Systems: Efficient Use of Swine and Feed Resources. Van Nostrand Reinhold, New York, NY.