
You've reached the Virginia Cooperative Extension Newsletter Archive. These files cover more than ten years of newsletters posted on our old website (through April/May 2009), and are provided for historical purposes only. As such, they may contain out-of-date references and broken links.
To see our latest newsletters and current information, visit our website at http://www.ext.vt.edu/news/.
Newsletter Archive index: http://sites.ext.vt.edu/newsletter-archive/

Enhanced Fertility in Boars Fed Diets Supplemented with Sel-Plex® Selenium
Authors: Drs. Mark J. Estienne and Allen F. Harper, Tidewater AREC and
Dr. James W. Knight and Ms. Susan M. Speight, Department of Animal & Poultry Sciences, Virginia Tech
Livestock Update, February 2009
Several research groups have conducted studies to investigate the effects of supplemental selenium on reproductive characteristics of boars (Segerson et al. 1981; Marin-Guzman et al., 1997, 2000a, 2000b; Jacyno, 2002; Kolodziej and Jacyno, 2005), and there is strong evidence to support the inclusion of this mineral in the daily ration. Improvements in sperm production, sperm morphology, and fertility have been reported for boars fed diets supplemented with inorganic selenium (sodium selenite), generally at levels of 0.5 ppm (Marin-Guzman et al., 1997, 2000a, 2000b). Because of environmental concerns, however, the Food and Drug Administration (FDA, 1987) allows a maximum of only 0.3 ppm supplemental selenium in swine diets. Mahan and Kim (1996) suggested that selenite may not be as biologically effective as the selenium indigenous in grains, which is incorporated in an organic form (selenomethionine). Sel-Plex® (Alltech, Inc.; Nicholasville, KY) is an organic source of selenium that consists primarily of selenomethionine. The working hypothesis in our laboratory is that because of greater “bio-availability”, semen quality and fertility in boars fed diets supplemented with 0.3 ppm selenium from an organic source will be superior to that produced from boars fed an equal amount of inorganic selenium.
The specific objectives of the research reported here were to: 1) evaluate sperm fertilizing capability for boars fed selenium from either organic or inorganic sources and, 2) determine if enhanced motility characteristics and fertility exhibited by spermatozoa collected from Sel-Plex-fed boars are maintained during long-term liquid storage in commercially-available extenders.
Following completion of the growing-finishing phase, boars were moved to the Research Boar Stud and were trained to mount an artificial sow and allow semen collection. Boars continued to receive, at a rate of 2.5 kg/day, a basal diet (n = 10) or the basal diet supplemented with 0.3 ppm selenium from either Sel-Plex (n = 10) or sodium selenite (n = 10).
Semen collection and analysis. Semen was collected using the gloved hand technique and was analyzed for volume, sperm concentration, total sperm cells, and characteristics of sperm motility as previously described (Estienne et al., 2007). Briefly, semen was filtered (US BAG, Minitube of America, Inc., Verona, WI) during collection to remove gel. Gel-free semen volume and gel weight were determined gravimetrically using a top-loading balance (Acculab; Minitube of America, Inc.). Semen samples were diluted in a commercially available extender (Androhep Lite; Minitube of America, Inc.) to achieve a ratio of semen to extender of 1:5. A computer-assisted sperm analysis system (Integrated Visual Optical System, Version 12; Hamilton Thorne Research, Beverly, MA) using starting values for boar sperm analysis consistent with manufacturer recommendations, was employed to determine sperm concentration and the following characteristics of sperm motility: the percentages of spermatozoa exhibiting motility and progressive motility, path velocity of the smoothed cell path (VAP), average velocity measured in a straight line from the beginning to the end of the sperm track (VSL), average velocity measured over the actual point to point track followed by the sperm cell (VCL), straightness (STR; average value of the ratio VSL/VAP) which measured the departure of the sperm cell path from a straight line, linearity (LIN; average value of the ratio VSL/VCL) which measured the departure of the cell track from a straight line, frequency with which the sperm track crossed the sperm path (i.e., frequency of sperm head crossing the sperm average path in either direction) (BCF), and amplitude of lateral head displacement corresponding to the mean width of the head oscillation as the sperm swam (ALH).
Determination of Sperm Fertilizing Capability. Previously described procedures from our laboratory (Whitaker and Knight, 2004) were used. Unless otherwise stated, chemicals used were purchased from Sigma Chemical Co. (St. Louis, MO). The in vitro fertilization (IVF) medium used was a modified Tris-buffered medium (mTBM) (Abeydeera and Day, 1997) containing 113.1 mM NaCl, 3 mM KCL, 7.5 mM CaCL2•2H20, 20 mM Tris, 11mM D(+)-glucose, 5mM sodium pyruvate, 1 mg/mL BSA (fraction V; 43H1097, initial fraction by heat shock) and 0.38 mg/mL caffeine. Medium was filtered through a 0.2 µm pore HT tuffryn membrane Acrodisc (Fisher Scientific, Pittsburgh, PA) and allowed to equilibrate at 39ºC in an atmosphere of 5% CO2.
Porcine oocytes were purchased from Bomed, Inc. (Madison, WI). Oocytes surrounded by a compact cumulus cell mass and uniform ooplasm were washed three times in a 50 mm x 9 mm Falcon polystyrene dish (Fischer Scientific; Pittsburgh, PA) using the North Carolina State University (NCSU) 23 maturation medium (Petters and Wells, 1993) and 100 oocytes were placed into each well of a Nunclon six well multidish (Fisher Scientific) containing equilibrated 500 µL of maturation medium overlaid with mineral oil (Specialty Media, Phillipsburg, NJ). The oocytes were incubated at 39ºC in an atmosphere of 5% CO2 for 48 hours.
After incubation, cumulus cells were removed by mixing oocytes with 0.1% hyaluronidase in NCSU 23 solution for 15-30 seconds. Oocytes were then washed three times in a 50 mm x 9 mm Falcon polystyrene dish in 100 µL drops of mTBM and stored in 50 µL drops of mTBM under mineral oil. Semen samples were centrifuged in 50 mL polypropylene conical tubes at 73 x g for 5 minutes. The supernatant was poured into a new tube and PBS containing 0.1% BSA added to bring the volume to 15 mL and centrifuged at 1052 x g for 5 minutes to collect viable sperm cells. The supernatant was then discarded and the pellet washed once more as described above. Following the wash, the supernatant was discarded and 1 mL of mTBM added to the pellet. The number of spermatozoa was then counted using a Bright-Line hemocytometer (Fisher Scientific). The spermatozoa were diluted with mTBM such that the final concentration was 1 x 106 spermatozoa/mL. Then, 50 µL of spermatozoa were added to each well, mixed, and the oocytes and spermatozoa were co-incubated at 39ºC in an atmosphere of 5% CO2 for 10 hours.
At the end of the IVF, oocytes were washed three times in 100 µL drops of PBS and then placed in 110 µL of PBS containing 10 µL of 1 mg/mL bisbenzimide H 33342 (Hoechst 33342) stain. After 10 minutes of staining, the oocytes were de-stained in PBS for 5 minutes and examined under a fluorescent microscope (346 nm excitation wavelength; 460 nm emission wavelength). Oocytes were characterized as whether or not they were penetrated by spermatozoa (swollen spermatozoon head), polyspermic (more than one swollen spermatozoon head), or undergoing male pronucleus (MPN) formation (visual identification of an MPN).
Statistical Analyses. Data were subjected to analyses of variance using the GLM procedure of SAS (SAS Institute Inc., Cary, NC). Individual means were compared using the PDIFF option of the GLM procedure.
Experiment 1 protocol: Effects of dietary supplementation with Sel-Plex on the maintenance of sperm motility and semen pH during storage
Ejaculates were collected from a total of 30 boars (n = 10/dietary treatment). Sub-samples of collected ejaculates were diluted in Beltsville Thawing Solution (BTS; IMV USA, Maple Grove, MN) and Androhep-Lite and stored in plastic AI bottles. Each bottle contained three billion spermatozoa in 85 mL of semen and extender. Semen bottles were stored in a semen storage unit (Minitube of America, Inc.) at 18º C for 10 days (day of collection = day 1). On each of days 1 through 10, an aliquot of each sample was removed and warmed to 37º C for 30 minutes. The sample was then analyzed for sperm motility characteristics as described above, and for pH (Orion 620; Fisher Scientific).
Experiment 2 protocol: Effects of dietary supplementation with Sel-Plex on the maintenance of fertility of boar spermatozoa during storage
Ejaculates were collected from a total of 18 boars (n = 6/dietary treatment). Semen was diluted in Androhep-Lite and stored as described above. Using IVF procedures outlined above, sperm fertilizing capability was determined on days 3 and 9 post-collection (day 1 = day of collection).
Experiment 1: Effects of dietary supplementation with Sel-Plex on the maintenance of sperm motility during storage
There were no three-way interactions between extender (BTS or Androhep-Lite), dietary treatment, and day (P > 0.1). Therefore, data for the different extenders were pooled within days. There were no effects (P > 0.1) of dietary treatment, day, or treatment x day for VCL. Other characteristics of sperm motility during storage for boars from the various treatment groups are depicted in Figure 1. The percentages of progressively motile sperm cells, VSL, and LIN decreased during storage (effect of day, P < 0.01) but were not affected (P > 0.1) by treatment or treatment x day. The percentage of motile spermatozoa (P < 0.01), VAP (P = 0.06), BCF (P = 0.04), and STR (P < 0.01) were affected by treatment x day. For the various characteristics of sperm motility, values of Sel-Plex-fed boars were in most cases, the greatest after 10 days of storage.
In commercial boar studs, sperm motility assessment is a technique used routinely for semen evaluation (Shipley, 1999). It is acknowledged, however, that like any on-farm laboratory test, assessment of sperm motility does not provide a completely accurate or quantitative measure of semen fertility per se. Visual motility assessment tends to be highly subjective and in many boar studs is being replaced with more objective, detailed and repeatable assessments using automated systems (Vyt et al., 2004b). Moreover, Holt et al. (1997) demonstrated that up to 24% of the variance in litter size due to boars on commercial swine farms could be explained by differences in sperm motion characteristics determined using CASA technology. Boar spermatozoa that exhibited increased VSL and track linearity were associated with larger litter sizes.
The precise mechanisms by which motility characteristics were enhanced in boars fed Sel-Plex compared with the other groups was beyond the scope of this investigation, but warrants further scrutiny. Marin-Guzman et al. (2000b) reported that selenium deficient boars produced spermatozoa with decreased ATP concentrations, and electron microscopy revealed that these cells had structural abnormalities to the tail midpiece, including altered mitochondrial shape and orientation and poor contact of the plasma membrane to the helical coil.
In the current investigation there was an effect of treatment x day (P < 0.01) for semen pH (Figure 2). Indeed, pH increased during storage in semen collected from control boars and boars fed a diet supplemented with selenite compared to Sel-Plex-fed boars. Whether the changes in pH during storage are the cause of, or are somehow related to, the differences in motility characteristics remains to be determined. However, in a study comparing five different commercial extenders for boar semen, pH of stored semen increased from day 1 to day 7, and pH was negatively correlated with sperm motility (Vyt et al., 2004a).
Experiment 2: Effects of dietary supplementation with Sel-Plex on the maintenance of fertility of boar spermatozoa during storage
Contained in table 1 are the data for the in vitro fertilization of oocytes by sperm cells collected from boars fed the various diets. On day 3 post semen collection, the number of monospermic fertilized oocytes tended to be greatest in the Sel-Plex group (P = 0.06) and fertilization rates were greater (P < 0.01) for the Sel-Plex individuals compared with the other two groups. In contrast the number of unfertilized oocytes was lesser in the Sel-Plex boars compared with controls or boars fed a diet supplemented with sodium selenite.
On day 9 post semen collection, fertilization rates tended to be greater (P = 0.08) for boars fed a diet supplemented with Sel-Plex compared with boars from the other two groups. The number of unfertilized oocytes tended (P = 0.10) to be lesser in Sel-Plex-fed boars compared to selenite-fed boars with the controls having an intermediate value that was not different from the other two groups.
Marin-Guzman et al. (1997) were the first to report effects of supplemental selenium on the actual fertility of boars. In their study, boars were supplemented with 0.5 ppm selenium and gilts were mated using AI with semen collected from experimental animals. When gilts were killed 5 to 7 days later, it was determined that selenium supplementation increased fertilization rates and the number of accessory spermatozoa. The results of the current study extend these findings and show that boars fed Sel-Plex at a level of 0.3 ppm have a greater fertilization rate than boars fed 0.3 ppm selenium from sodium selenite or un-supplemented control boars. Moreover, the enhanced fertility appears to be maintained during long-term liquid storage.
Estienne, M.J., A.F. Harper, and J.L. Day. 2007. Characteristics of sperm motility in boar semen diluted in different extenders and stored for seven days at 18ºC. Reprod. Biol. 7:221-231.
FDA, 1987. Food additives permitted in feed and drinking water of animals: Selenium. Fed. Reg. 52:10887.
Holt, C., W.V. Holt, H.D.M. Moore, H.C.B. Reed, and R.M. Curnock. 1997. Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: results of two fertility trials. J. Andrology 18:312-323.
Jacyno, E., M. Kawecka, M. Kamyczek, A. Kolodziej, J. Owsianny, and B. Delikator. 2002. Influence of inorganic SE + vitamin E and organic SE + vitamin E on reproductive performance of young boars. Agric. Food. Sci. Finland. 11:175-184.
Kolodziej, A. and E. Jacyno. 2005. Effect of selenium and vitamin E supplementation on reproductive performance of young boars. Archiv fur Tierzucht 48:68-75.
Mahan, D.C., and Y.Y. Kim. 1996. Effect of inorganic or organic selenium at two dietary levels on reproductive performance and tissue selenium concentrations in first-parity gilts and their progeny. J. Anim. Sci. 74:2711-2718.
Marin-Guzman, J., D.C. Mahan, Y.K. Chung, J.L. Pate, and W.F. Pope. 1997. Effects of dietary selenium and Vitamin E on boar performance and tissue responses, semen quality, and subsequent fertilization rates in mature gilts. J. Anim. Sci. 75:2294-3003.
Marin-Guzman, J., D.C. Mahan, and J.L. Pate. 2000a. Effect of dietary selenium and vitamin E on spermatogenic development in boars. J. Anim. Sci. 78:1537-1543.
Marin-Guzman, J., D.C. Mahan, and R. Whitmoyer. 2000b. Effect of dietary selenium and vitamin E on the ultrastructure and ATP concentration of boar spermatozoa, and the efficacy of added sodium selenite in extended semen on sperm motility. J. Anim. Sci. 78:1544-1550.
National Research Council. 1998. Nutrient requirements of swine, 10th ed. Washington, DC: National Academy Press.
Petters, R.M., and C.D. Wells. 1993. Culture of pig embryos. J. Reprod. Fertil. Suppl. 48:61-73.
Segerson, E.C., W.R. Getz, and B.H. Johnson. 1981. Selenium and reproductive function in boars fed a low selenium diet. J. Anim. Sci. 53:1360-1367.
Vyt, P., D. Maes, E. Dejonckheere, F. Catryck, and A. Van Soom. 2004a. Comparative study on five different commercial extenders for boar semen. Reprod. Domest. Anim. 39:8-12.
Vyt, P., D. Maes, T. Rijsselaere, E. Dejonckheere, F. Catryck, and A. Van Soom. 2004b. Motility assessment of porcine spermatozoa: a comparison of methods. Reprod. Domest. Anim. 39:447-453.
Whitaker, B.D. and J.W. Knight. 2004. Exogenous γ-glutamyl cycle compounds supplemented to in vitro maturation medium influence in vitro fertilization, culture, and viability of porcine oocytes and embryos. Theriogenology 62:311-322.
| Table 1. Effects of dietary supplementation with an organic (Sel-Plex; Alltech, Inc., Nicholasville, KY) or inorganic (Sodium Selenite) source of selenium on in-vitro fertilization boar spermatozoa at days 2 and 8 after semen collection. |
|---|
| Sel-Plex | Sodium Selenite | No supplement | SE | P | ||
|---|---|---|---|---|---|---|
| Number of boars | 6 | 6 | 6 | --- | --- | |
| Day 2 after semen collection | ||||||
| Total oocytes | 92.8 | 85.3 | 95.7 | 4.4 | 0.27 | |
| Unfertilized | 20.7a | 28.0b | 29.7b | 1.7 | <0.01 | |
| Monospermic | 55.2c | 43.5d | 51.2c | 3.1 | 0.06 | |
| Polyspermic | 17.0 | 13.8 | 14.8 | 2.1 | 0.56 | |
| Male pronucleus formation | 67.7 | 56.1 | 61.7 | 3.6 | 0.12 | |
| Fertilization rate, % | 77.8a | 67.4b | 68.5b | 2.0 | <0.01 | |
| Polyspermy rate, % | 18.3 | 16.3 | 15.0 | 2.0 | 0.58 | |
| Day 8 after semen collection | ||||||
| Total oocytes | 86.0 | 91.3 | 89.5 | 4.1 | 0.66 | |
| Unfertilized | 32.2e | 47.7f | 42.7e | 4.7 | 0.10 | |
| Monospermic | 43.0 | 34.2 | 39.0 | 3.6 | 0.27 | |
| Polyspermic | 10.8 | 9.5 | 7.8 | 1.6 | 0.46 | |
| Male pronucleus formation | 53.0 | 43.5 | 43.5 | 4.9 | 0.33 | |
| Fertilization rate, % | 63.5e | 49.5f | 53.3f | 4.1 | 0.08 | |
| Polyspermy rate, % | 12.9 | 10.3 | 8.8 | 1.6 | 0.25 | |
| a,b Values within a row with different superscripts differ (P < 0.01). |
| c,d Values within a row with different superscripts tended to differ (P < 0.06). |
| e,f Values within a row with different superscripts tended to differ (P < 0.10). |
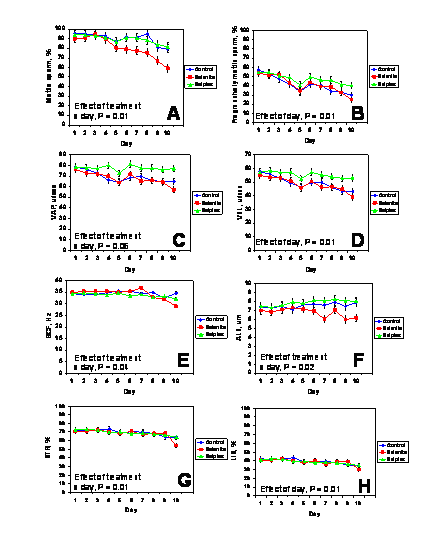
Figure 1. Characteristics of sperm motility for semen collected from control boars and boars fed diets supplemented with Sel-Plex or sodium selenite (n = 10/dietary treatment). Semen was extended and stored for 10 days at 18ºC. The percentage of progressively motile (panel B) sperm cells, average velocity measured in a straight line from the beginning to the end of sperm track (VSL; panel D), and linearity, which was the average value of the ratio VSL/VCL and measured the departure of the cell track from a straight line (LIN; panel H) were affected by day (P < 0.01). The percentage of motile sperm cells (P < 0.01; panel A), the path velocity of the smoothed cell path (VAP; P = 0.06 panel C), the frequency with which the sperm track crossed the sperm path (BCF; P = 0.04; panel E), the amplitude of lateral head displacement corresponding to the mean width of the head oscillation as the sperm swam (ALH; P = 0.02; panel F), and straightness, which was the average value of the ratio VSL/VAP and measured the departure of the cell path from a straight line (STR; P < 0.01; panel G), were affected by treatment x day.
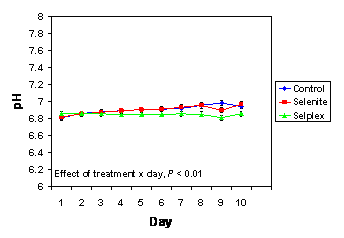
Figure 2. The pH of semen that was collected from boars fed diets supplemented with Sel-Plex or sodium selenite or that received no selenium supplementation (n = 10/dietary treatment), and was extended and stored at 18ºC for 10 days. There was an effect (P < 0.01) of treatment x day.