
You've reached the Virginia Cooperative Extension Newsletter Archive. These files cover more than ten years of newsletters posted on our old website (through April/May 2009), and are provided for historical purposes only. As such, they may contain out-of-date references and broken links.
To see our latest newsletters and current information, visit our website at http://www.ext.vt.edu/news/.
Newsletter Archive index: http://sites.ext.vt.edu/newsletter-archive/

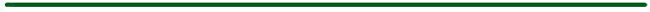
Figure 1. Illustration of the excitation and emission of heat and fluorescence by OBs.
(Courtesy of R. David Holbrook, National Institute of Standards and Technology)
OBs can be classified, based on structure and properties, into some 11 major chemical families, each containing numerous sub-families, hundreds of compounds, and thousands of different formulations (2). All OBs are highly-substituted ring (aromatic) structures that contain many double bonds that can be activated by UV light. Thousands of OB formulations have been evaluated by the detergent industry, but relatively few have met the requirements of that industry and have been added to detergents as fluorescent whitening agents (FWAs). As well as detergents, OBs are used in textiles, many types of papers, plastics, and synthetic fibers, plus widespread use in some medical, chemical, and petroleum applications. The chemical families that include the OB formulations that are most widely used by the detergent industry are the carbocycles (mainly the distyrylbiphenyls), and the triazinylaminostilbenes (2).
Nearly all modern laundry detergents contain FWAs and they are discharged in substantial quantities with household wastewater where they can serve as an indicator of failing onsite septic systems (1, 3, 4, 5). Laundry effluent is also associated with sanitary wastewaters, so FWAs can also serve as a surrogate of sanitary wastewater in storm drainage outfalls and receiving waters (6). As FWAs have a propensity to adsorb to fabric, a significant proportion of FWA in an effluent or in a receiving water will be associated with colloidal and particulate matter rather than in the aqueous phase. However, FWAs photodecay and biodegrade relatively slowly, allowing them to serve as ideal indicators of illicit discharges or accidental cross-connections into storm sewers (6) or septic tank contamination in shallow groundwater (7). Although FWA detection can be more problematic if laundry facilities are absent (commercial buildings, some homes, public restrooms, etc.), detection is still possible as some (but not all) handwashing or dishwashing detergents contain FWAs, as do essentially all toilet papers (Figure 2). Toilet paper breaks down rapidly (even in distilled water, bottle on the right in Figure 2), releasing the FWAs into the water where they can be detected and measured with a fluorometer (3, 5).

Figure 2. Fluorescence as a result of FWAs in commercial toilet paper.
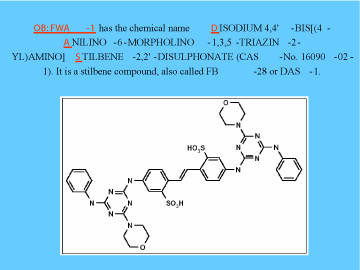
Figure 3. Structure of FWA-1 (DAS-1), used extensively in detergents throughout the world (8).
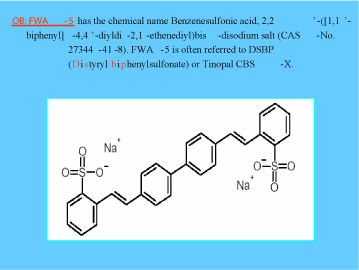
Figure 4. Structure of FWA-5 (tinopal CBS-X), an example of a widely-used class of FWAs (9).
Both compounds are highly soluble, behave like colorless cotton dyes, and have a high affinity for cotton and synthetic fibers. In detailed exposure studies, neither compound was found to pose any health risks to primary consumers. In the U.S. some 97% of laundry detergents contain one (or sometimes both) of the compounds in Figures 3 and 4. In New Zealand, FWA-5 (tinopal CBS-X) was found to be the sole FWA in nearly 100% of all detergents tested (10), while studies on Tokyo Bay indicated that both the stilbene- and DSPB-types were contained in detergents in Japan (11).
One of the most popular approaches to FWA detection has been the use of cotton pads that are placed in receiving waters for several days, then collected and tested for fluorescence with a hand-held UV light or an inexpensive UV source such as the transilluminator in Figure 2 (6, 12). The obvious advantages to this approach include instantaneous detection, inexpensive equipment, lack of formal training needed for operators, and ease of examining large numbers of samples in a short period of time (including detection in the field). The disadvantages include lack of accuracy (inability to distinguish between FWAs and other fluorescent compounds), limitations of the presence-absence approach where concentrations cannot be readily obtained, and lack of sensitivity. This approach only works if the cotton pads are very close to the source of the FWAs where the concentrations are sufficiently high to result in detection of fluorescence. However, the lack of sensitivity with this approach can result in meaningful concentrations of FWAs that are present but undetectable by a hand-held UV light and the unaided eye (Figure 5).

Figure 5. Detection of FWA in a standard (left) and serial dilutions by a hand-held UV light. The flask on the right does not fluoresce, but contains a concentration of FWA that is representative of septic tank effluent.
More accurate fluorometric methods involve use of an instrument where some level of quantification of both the excitation source (R) and the emission detector (S) can be obtained (Figure 6). Advantages to this approach include instantaneous detection (once samples are transported to the lab), ease of examining large numbers of samples in a short period of time, greater accuracy (ability to distinguish between FWAs and other fluorescent compounds), elimination of the presence-absence approach so that some quantification of concentrations can be obtained, and better sensitivity (detection of lower concentrations). The disadvantages include expense of equipment (typical lab fluorometers range from a few thousand to over eighteen thousand dollars), and higher formal training or education needed for operators. The more accurate and sensitive the fluorometer, the more it will cost. High-end units are capable of measuring ug/L amounts. One of the more useful fluorometers has proven to be the Turner Designs (Sunnyvale, CA) Model AU-10 (13, Figure 7).
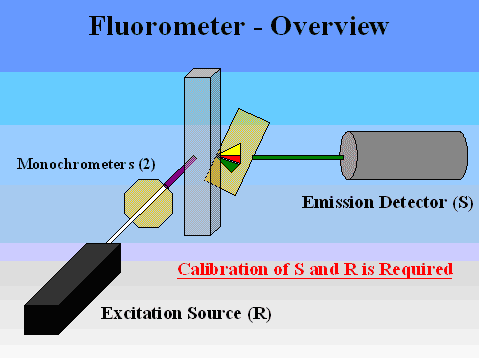
Figure 6. Overview of a fluorometer, illustrating excitation and emission detection.
(Courtesy of R. David Holbrook, National Institute of Standards and Technology)
The remaining method involves direct chemical measurement of the FWAs rather than detection by fluorescence. Such measurements are obtained via high performance liquid chromatography (HPLC, 1, 5, 10, 11). This is the most expensive approach and HPLC facilities can cost many tens of thousands of dollars to establish, maintain, and operate. Usually a dedicated technician with specialized training is also required. Accuracy and sensitivity are both excellent, but the same levels can also be obtained with high-end fluorometers. No HPLC system is suitable for field deployment and detection in any single sample is not rapid. For most applications, the expense and time required for analysis eliminates HPLC from consideration in projects where large numbers of samples will be collected and analyzed, unless a dedicated facility is available at a reasonable cost.

Figure 7. The Turner AU-10 fluorometer, with cuvette assembly. The instrument is armored for field use, can be operated with a car battery, and operated in the field either in cuvette mode or continuous flow, where a continuous measurement assembly is interchanged with the cuvette kit (13).
1 This article is Part I of a 3-part series. Part II describes case studies where the detection of OBs was deployed in the field to locate leaking on-site wastewater treatment systems and Part III describes case studies where OB detection was used to find cross-connected sewer lines and storm drains in urban coastal environments.
Visit Virginia Cooperative Extension